Please click a title to open the tab
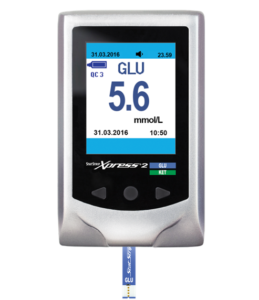
Measurement of blood glucose using meters and strips, either by diabetic patients themselves of by nursing staff, is a well-established procedure.
Whole blood ketone monitoring is important for managing adult and paediatric patients with diabetes ketoacidosis (DKA), metabolic disease, managing fasting regimes or ketogenic diets, and managing alcoholic ketoacidosis.
The Nova StatStrip Xpress®2 blood glucose and ketone meters are light portable meters that provides accurate results in seconds. When a drop of blood is placed on the Nova StatStrip® Glucose or Nova StatStrip® β-Ketone test strips, the analyte in the blood reacts with the chemicals on the test strip, producing a small electrical current. The current is measured and then a result is displayed by the Nova StatStrip Xpress®2 meter. The size of the current depends on the amount of glucose or β-ketone in the blood sample.
IMPORTANT: There are two separate strip types for glucose or β-ketone. Please ensure the correct strip type is used for the test required.
For further details, training information and copies of key documents contact us or go to the Policies and Procedures tab.

Measurement of blood glucose using meters and strips, either by diabetic patients themselves of by nursing staff, is a well-established procedure.
Whole blood ketone monitoring is important for managing adult and paediatric patients with diabetes ketoacidosis (DKA), metabolic disease, managing fasting regimes or ketogenic diets, and managing alcoholic ketoacidosis.
The Nova StatStrip Xpress®2 blood glucose and ketone meters are light portable meters that provides accurate results in seconds. When a drop of blood is placed on the Nova StatStrip® Glucose or Nova StatStrip® β-Ketone test strips, the analyte in the blood reacts with the chemicals on the test strip, producing a small electrical current. The current is measured and then a result is displayed by the Nova StatStrip Xpress®2 meter. The size of the current depends on the amount of glucose or β-ketone in the blood sample. This meter will connect to the electronic patient record and prevent the need for manual result transcription.
IMPORTANT: There are two separate strip types for glucose or β-ketone. Please ensure the correct strip type is used for the test required.
For further details, training information and copies of key documents contact us or go to the Policies and Procedures tab.

Siemens Multistix 8 SG urine strips provide screening tests for glucose, ketones, blood, pH, protein, nitrite, leucocytes and specific gravity. The reagent test areas on the strips are ready to use upon removal from container and can be read visually.
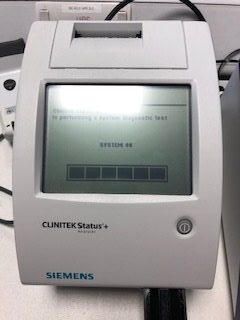

The Siemens CLINITEK Status+ device automatically times and reads the Siemens Multistix 8 SG urine screening sticks, reducing the risk of erroneous results being reported.
For further details, training information and copies of key documents contact us or go to the Policies and Procedures tab.

Clearview EASY HCG pregnancy test is a rapid diagnostic device for the qualitative detection of human Chorionic Gonadotrophin (HCG) in urine. HCG is a glycoprotein hormone produced by blastocysts. HCG normally begins to be detected in the urine from 7 days after conception. The presence and rapid rise of HCG levels in urine make it an excellent marker for the diagnosis of pregnancy.
For further details, training information and copies of key documents contact us or go to the Policies and Procedures tab.

Locations and tests available (X = available, Blank = not available):
| MEASURED PARAMTERS AVAILABLE | DERIVED PARAMETERS AVAILABLE | ||||||||||||||||||||
| Location | Analyser Name | Analyser Serial Number | Panels available | pH | pCO2 | pO2 | Na+ | K+ | Cl- | Ca++ | Hct | Glu | Lac | tHb | COHb | Met Hb | BE(B) | CA++ (7.4) | sO2 | HCO3-std | HCO3- c |
| AMU- MTU POCT room | 1-AMU | 16030248 | Arterial, Venous, Capillary, Micro, Other (pH), Carboxy | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| AMU- SDEC | 15-AMU 2 SDEC | 20116436 | Arterial, Venous, Capillary, Micro, Other (pH), Carboxy | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| Creedy | 2- CREEDY | 16040272 | Arterial, Venous, Capillary, Micro | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Yealm | 3- YEALM | 16040271 | Arterial, Venous, Capillary, Micro, Other (pH) | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Culm | 4- CULM | 20034173 | Arterial, Venous, Capillary, Micro, Carboxy | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| ED RESUS | 5- ED | 16030249 | Arterial, Venous, Capillary, Micro, CarboxyMetHb | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||
| ITU 1 | 6-ITU1 | 16020235 | Arterial, Venous, Capillary, Micro | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| THEATRES | 11- THEATRE RECOVERY | 16030250 | Arterial, Venous, Capillary, Micro | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| ITU 2 | 7-ITU 2 | 20013927 | Arterial, Venous, Capillary, Micro | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | |||
| SAU ABBEY | 10- SAU ABBEY | 20044350 | Arterial, Venous, Capillary, Micro | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| PEOC | 9- PEOC | 20044419 | Arterial, Venous, Capillary, Micro | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Labour One | 13-LABOUR1 | 16020236 | cordarterial, cordvenous, Arterial, Venous, FBS | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Labour Two (blue) | 12- LAB 2 BLUE | 16030251 | cordarterial, cordvenous, Arterial, Venous, FBS | X | X | X | X | X | |||||||||||||
| NNU | 14- NNU | 16030237 | Arterial, Venous, Capillary, Micro | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
| Nightingale | 8- NIGHTINGALE | 20034243 | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | ||||
X = available, blank = not available

Location:
The foetal fibronectin test is to be used as an aid in assessing the risk of preterm delivery in less than or equal to 7 or 14 days from the time of cervicovaginal sample collection in pregnant women with signs and symptoms of early preterm labor, intact amniotic membranes and minimal cervical dilatation (< 3 cm), sampled between 24 weeks, 0 days and 34 weeks, 6 days gestation.
The foetal fibronectin test is further indicated for use in conjunction with other clinical information as an aid in assessing the risk of preterm delivery in less than or equal to 34 weeks, 6 days when a cervicovaginal sample is obtained during a routine prenatal visit between 22 weeks, 0 days and 30 weeks, 6 days of gestation in women with a singleton pregnancy.
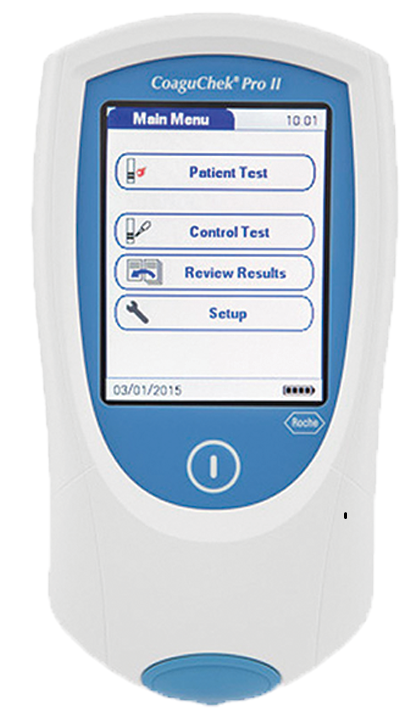
The Roche CoaguChek XS devices are used to monitor INR levels in patients who are taking warfarin. It is important for a patient to be prescribed sufficient warfarin to ensure blood clots do not form but equally important to ensure that too much warfain is not prescribed which could lead to excessive bleeding.
The CoaguChek system is intended for use by professional healthcare providers for quantitative prothrombin time for the monitoring of warfarin therapy (INR). The CoaguChek system uses fresh capillary or non-coagulated venous whole blood.
The INR is calculated from the prothrombin time; the test strip contains human thromboplastin; when whole blood is applied to the test strip clotting is initiated. This process generates thrombin which reacts with other chemicals in the strip to produce an electrochemical signal. This signal is converted by an algorithm encoded in the code chip into the INR reading displayed on the screen.
There are various models in use across the Trust and community sites including the XS, XS Plus and the Pro / Pro II models. The Pro II is the newest model and has enhanced connectivity which allows for patient results to be transmitted to patient eNotes.
Test strips and quality control material are available to purchase directly through the Trust procurement system; care should be taken to ensure the correct strips are purchased.
For further information details please the laboratory.
For maintenance, training & troubleshooting please contact Roche directly https://diagnostics.roche.com/global/en/contact-forms/contact-us-support.html

Location:
The HemoCue 201+ meters are used to measure haemoglobin and so help assess blood loss.
The Hemocue Hb 201 system provides lab accuracy and ease of use for point-of-care Haemoglobin measurement.
The system utilises an oxidation method to quantify haemoglobin concentration in around 15-45 seconds. Results are displayed as a digital reading in g/l. Newer models have connectivity capabilities allowing for results to be recorded electronically to patient eNotes.
When a drop of blood is applied to a ‘microcuvette’ a reaction takes place between the blood and chemicals in the microcuvette. A change in light absorbance is read by the device and the Hb concentration calculated.
For further enquiries e.g. training, ordering, maintenance or trouble shooting please contact the Haematology laboratory.
TEG 6s
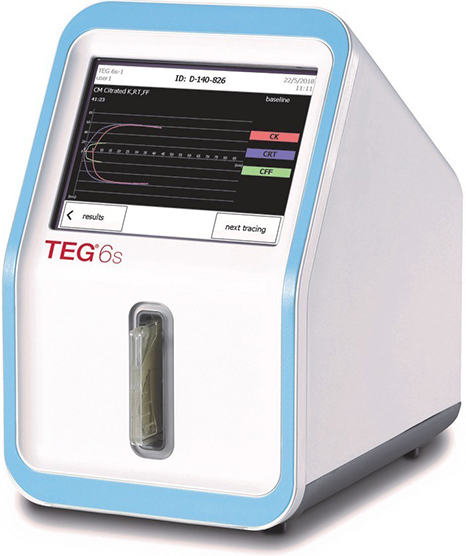
Haemonetics’ TEG® 6s Hemostasis Analyzer provides rapid, comprehensive and accurate identification of an individual’s hemostasis condition.
Viscoelastic testing assesses the dynamic mechanical properties of a clot during its initiation, amplification and propagation and the finishing fibrinolysis. The TEG 6s device is a non-invasive diagnostic instrument designed to monitor and analyse the coagulation state of a blood sample in order to assist in the assessment of patient clinical haemostasis conditions during surgery and/or significant blood loss.
With LED illumination, a detector measures up/down motion of the blood meniscus. The frequency leading to resonance is identified and then converted to the TEG system readout.
The TEG 6s is indicated for use with adult patients. Results from the TEG 6s should not be the sole basis for patient diagnosis, but should be considered together with the patient’s medical history, the clinical picture and, if necessary, other coagulation tests.
For further information or details please contact the laboratory.
For troubleshooting and maintenance please contact Haemonetics https://teg.haemonetics.com/en-gb/contact-us

Unistik 3 has been developed to provide the most comfortable blood sampling experience.
Patented Comfort Zone Technology® gives a virtually pain free experience. The unique raised dots of Comfort Zone Technology® mask the sensation of pain by stimulating the nerve endings responsible for contact, pressure and texture, making the procedure a more comfortable experience.
Unistik 3 allows the user to decide exactly when to activate the device and how much pressure to apply. The design makes it easy and comfortable to grip, giving the user complete confidence and control during the procedure.

Location:
The Nova StatSensor® eGFR Meter is used for patients having MRI scans. Some patients having an MRI require an injection of contrast media, due to the toxicity of the contrast media these patients will need to have a recent eGFR result. When the eGFR is not available, this device can be used to measure the eGFR.
For further details, training information and copies of key documents contact us or go to the Policies and Procedures tab.

Location:
Activated Clotting Time (ACT) tests are general coagulation screening tests that are used to measure the functionality of the blood coagulation cascade. It is used to assess heparin therapy during cardiac procedures such as Percutaneous Coronary interventions (PCI), Cardiac Electrophysiology procedures (EP’s) and for patients on Cardiac Bypass. Measurement of ACT is required to ensure that the optimum dose of heparin is administered to the patient during and prior to procedures.
The GEM® Hemochron100 ACT-LR cartridges provide quantitative assays for monitoring heparin anticoagulation during medical procedures.
For further details, training information and copies of key documents contact us or go to the Policies and Procedures tab.
EXCELLING IN SCIENCE & SERVICE



